|
|
|
|
Definition
|
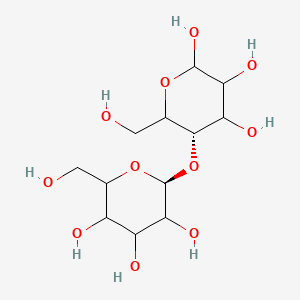
A natural carbohydrate high polymer (polysaccha- ride) consisting of anhydroglucose units joined by an oxygen linkage to form long molecular chains that are essentially linear. It can be hydrolyzed to glucose. The degree of polymerization is from 1000 for wood pulp to 3500 for cotton fiber, giving a molecular weight from 160,000 to 560,000. Cellulose is a colorless solid, d approximately 1.50, insoluble in water and organic solvents. It will swell in sodium hydroxide solution and is soluble in Schweitzer’s reagent. It is the fundamental con- stituent of all vegetable tissues (wood, grass, cotton, etc.) and the most abundant organic material in the world. Cotton fibers are almost pure cellulose; wood contains approximately 50%. The physical structure of cellulose is unusual in that it is not a single crystal but consists of crystalline areas embedded in amorphous areas. Chemical reagents penetrate the latter more easily than the former. Cel- lulose is virtually odorless and tasteless and is com- bustible, with an ignition point of approximately 450F. In some forms, it is flammable. For example, railroad shipping regulations require a flammable label on such items as burnt fiber, burnt cotton, wet waste paper, and wet textiles. Fires have been known to occur in warehouses in which telephone books were stored. These were undoubtedly due to heat buildup in the paper caused by microbial activity and self-sustaining oxidation.
|
|
Production Methods
|
Microcrystalline cellulose and carboxymethylcellulose sodium is a spray- or bulk-dried blend of microcrystalline cellulose and sodium carboxymethylcellulose. It is prepared by the chemical depolymerization of highly purified wood pulp. The original crystalline areas of the pulp fibers are combined with sodium carboxymethylcellulose, which serves as a protective colloid and also facilitates dispersion of the product; it is then either spray- or bulk-dried.
|
|
General Description
|
Odorless, white powdery fibers. Density 1.5 g cm-3. The biopolymer composing the cell wall of vegetable tissues. Prepared by treating cotton with an organic solvent to de-wax Cellulose microcrystalline and removing pectic acids by extration with a solution of sodium hydroxide. The principal fiber composing the cell wall of vegetable tissues (wood, cotton, flax, grass, etc.). Technical uses depend on the strength and flexibility of its fibers. Insoluble in water. Soluble with chemical degradation in sulfuric aicd, and in concentrated solutions of zinc chloride. Soluble in aqueous solutions of cupric ammonium hydroxide (Cu(NH3)4(OH)2).
|
|
Reactivity Profile
|
Cellulose microcrystalline is combustible. Incompatible with strong oxidizing agents including bromine pentafluoride, sodium nitrate, fluorine, perchlorates, perchloric acid, sodium chlorate, magnesium perchlorate, F2, zinc permanganate, sodium nitrite, sodium nitrate, sodium peroxide. Nitration with a mixture of nitric and sulfuric acids produces Cellulose microcrystalline nitrates (celluloid pyroxylin, soluble pyroxyline, guncotton) which are flammable or explosive.
|
|
Health Hazard
|
Cellulose is inert and is classified as a nuisance dust. It has little, if any, adverse effect on the lung, and there are no reports of organic disease or toxic effect. The health effects attributed to wood, cotton, flax, jute, and hemp are not attributable to their cellulose content but rather to the presence of other substances. Cellulose fibers were found in the blood and urine of human volunteers fed dyed cellulose; there were no ill effects.
|
|
Pharmaceutical Applications
|
Microcrystalline cellulose and carboxymethylcellulose sodium is used to produce thixotropic gels suitable as suspending vehicles in pharmaceutical and cosmetic formulations. The sodium carboxymethylcellulose aids dispersion and serves as a protective colloid. Concentrations of less than 1% solids produce fluid dispersions, while concentrations of more than 1.2% solids produce thixotropic gels. When properly dispersed, it imparts emulsion stability, opacity and suspension in a variety of products, and is used in nasal sprays, topical sprays and lotions, oral suspensions, emulsions, creams and gels.
|
|
Industrial uses
|
Cellulose is the main constituent of the structureof plants (natural polymer) that, whenextracted, is employed for making paper,plastics, and in many combinations. Celluloseis made up of long-chain molecules inwhich the complex unit C6H10O5 is repeatedas many as 2000 times. It consists of glucose molecules with three hydroxyl groups foreach glucose unit.One of the simplest forms of cellulose usedindustrially is regenerated cellulose, in whichthe chemical composition of the finished productis similar to that of the original cellulose. Itis made from wood or cotton pulp digested ina caustic solution. Cellophane is a regeneratedcellulose in thin sheets for wrapping and otherspecial uses include windings on wire andcable.
|
|
Biochem/physiol Actions
|
Cellulose helps in maintaining the structural stability of plant cell walls. It is an important component of paper and fabrics made from cotton, and linen.
|
|
Safety
|
Microcrystalline cellulose is widely used in oral pharmaceutical formulations and food products and is generally regarded as a relatively nontoxic and nonirritant material. Microcrystalline cellulose is not absorbed systemically following oral administration and thus has little toxic potential. Consumption of large quantities of cellulose may have a laxative effect, although this is unlikely to be a problem when cellulose is used as an excipient in pharmaceutical formulations. Deliberate abuse of formulations containing cellulose, either by inhalation or by injection, has resulted in the formation of cellulose granulomas.
|
|
storage
|
Microcrystalline cellulose and carboxymethylcellulose sodium is hygroscopic and should not be exposed to moisture. It is stable over a pH range of 3.5–11. Store in a cool, dry place. Avoid exposure to excessive heat.
|
|
Incompatibilities
|
Microcrystalline cellulose is incompatible with strong oxidizing agents.
|
|
Regulatory Status
|
Microcrystalline cellulose and carboxymethylcellulose sodium is a mixture of two materials both of which are generally regarded as nontoxic: Microcrystalline cellulose GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (inhalations; oral capsules, powders, suspensions, syrups, and tablets; topical and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. Carboxymethylcellulose sodium GRAS listed. Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (dental preparations; intra-articular, intrabursal, intradermal, intralesional, and intrasynovial injections; oral drops, solutions, suspensions, syrups and tablets; topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
|